Understanding the pH Level in Food Preservation Methods

What is pH and Why Does it Matter in Food?
pH is a scale that measures how acidic or alkaline a substance is, ranging from 0 to 14. In food, the pH level can influence flavor, texture, and preservation. Foods with low pH (acidic) tend to resist spoilage, while those with high pH (alkaline) are more prone to bacterial growth and spoilage. Understanding pH helps us choose the right preservation methods for different foods.
The importance of pH in food preservation cannot be overstated; it is the key to safe and delicious food.
For example, tomatoes have a pH range of about 4.0 to 4.6, making them acidic enough to be safely canned without excessive processing. This is crucial for ensuring that harmful bacteria do not thrive. In contrast, high-pH foods like certain vegetables might require additional acidification to ensure they can be safely preserved, illustrating how pH directly impacts food safety.
In essence, knowing the pH of our food helps guide our preservation choices, ensuring that we can enjoy our favorite flavors safely and for longer periods.
How pH Levels Affect Food Preservation Methods
Different food preservation methods rely on the acidity of the food to inhibit spoilage. For instance, canning often requires a certain pH level to ensure safety from botulism, a dangerous bacteria. Foods with a pH below 4.6 are generally safe to process in a water bath canner, while those above that threshold often require pressure canning, which raises the temperature to kill harmful organisms.
Fermentation is another method that takes advantage of pH levels. During fermentation, beneficial bacteria produce lactic acid, lowering the pH and creating an environment that inhibits spoilage organisms. Think of yogurt or pickles; their tangy taste comes from this natural acidification process that also preserves the food.
pH Affects Food Safety and Preservation
Understanding pH levels helps determine safe preservation methods, reducing the risk of spoilage and harmful bacteria.
In summary, the pH level of food plays a critical role in determining which preservation methods are safe and effective. By understanding this connection, we can make informed choices when preserving our food.
The Role of Acid in Food Safety and Preservation
Acids play a pivotal role in food preservation, acting as natural preservatives that inhibit the growth of harmful bacteria. When we add vinegar to vegetables or citrus to meats, we're lowering the pH and making the environment less hospitable for pathogens. For example, pickling cucumbers in a vinegar brine not only enhances flavor but also ensures they remain safe to eat over time.
Understanding the pH of food can empower you to preserve flavors and textures while ensuring safety.
Moreover, the effectiveness of acids can vary depending on the type of food and its inherent pH. For instance, while vinegar is great for pickling, citrus juice may not be acidic enough for preserving certain low-acid foods. Therefore, knowing the right type of acid to use based on the food’s characteristics is crucial for safe preservation.
Ultimately, understanding how acids affect pH levels and food safety allows us to preserve our meals confidently, ensuring they remain delicious and safe for consumption.
Common Food Preservation Techniques and Their pH Requirements
Several popular food preservation techniques hinge on pH levels. For instance, canning, freezing, drying, and fermenting each has its own pH requirements to ensure safety and effectiveness. Water bath canning is suitable for high-acid foods like jams and jellies, while pressure canning is necessary for low-acid foods like green beans.
Another technique, fermentation, not only preserves food but also enhances its flavors and nutritional profile. The pH of the food must drop sufficiently during fermentation to ensure pathogens cannot survive. For example, sauerkraut relies on lactic acid bacteria to lower the pH, creating a tangy product that lasts for months.
Acids Enhance Flavor and Safety
Adding acids like vinegar or citrus not only preserves food but also enhances its flavor while ensuring safety.
In short, each preservation method has specific pH requirements, and understanding these can help you choose the right technique for your food items.
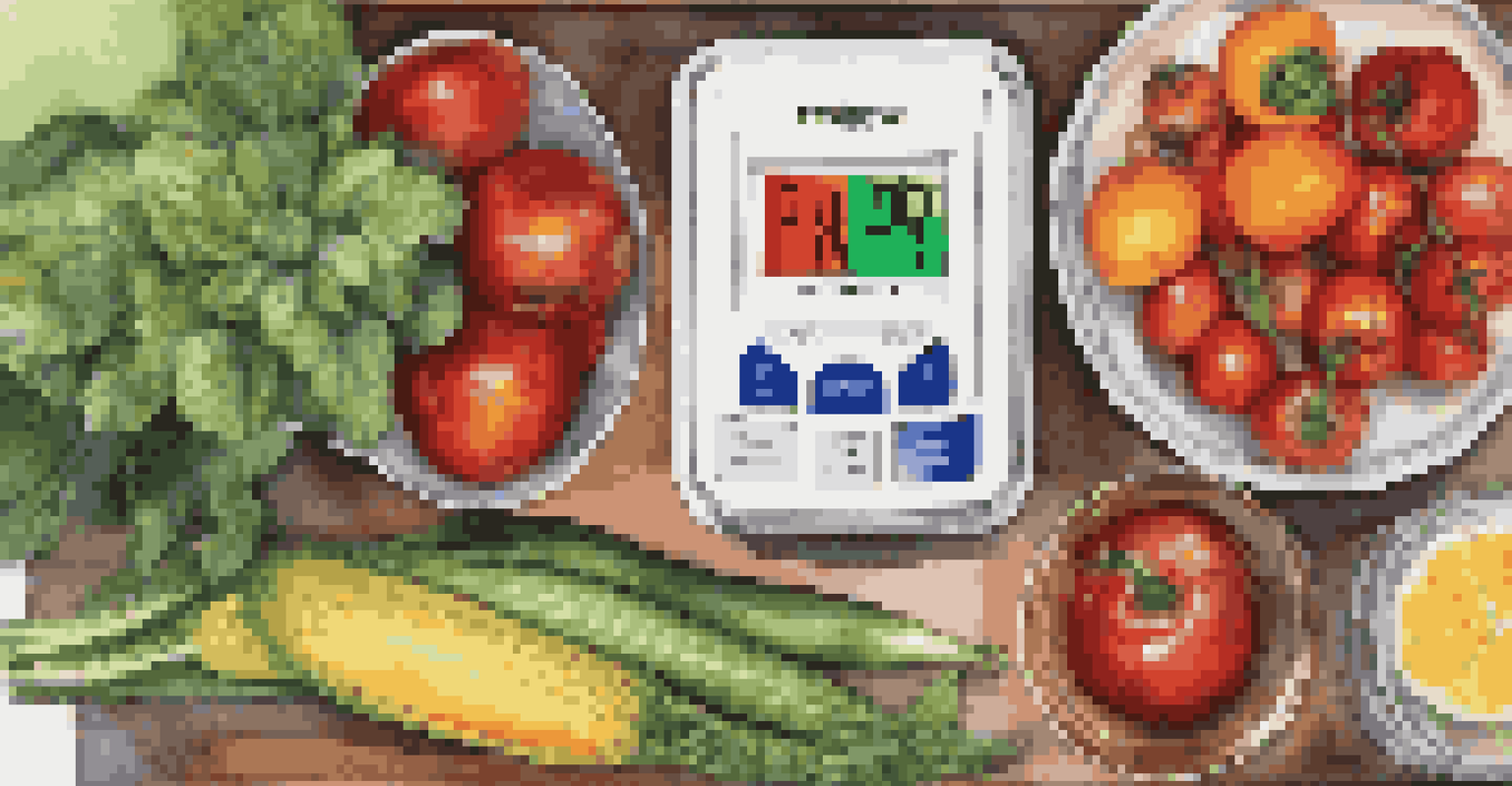
Testing and Measuring pH in Foods
To ensure food preservation methods are effective, measuring pH is essential. You can use pH strips or digital pH meters, both of which provide a quick and reliable way to assess acidity levels. This is particularly important when canning or fermenting, as the safety of these processes often hinges on achieving the right pH.
For instance, if you're making homemade salsa, testing the pH can help determine whether it's safe to water bath can. Ideally, the pH should be below 4.6 for safe preservation. If your salsa is too high, you might need to add more acid, like lemon juice or vinegar, to ensure safety.
Ultimately, regularly testing pH not only enhances food safety but also helps you refine your preservation techniques for better results.
The Impact of pH on Flavor and Texture in Preserved Foods
Beyond safety, pH levels also influence the flavor and texture of preserved foods. For example, the tanginess of pickled vegetables comes from the acetic acid in vinegar, which not only preserves but also enhances taste. Similarly, the fermentation process creates a unique flavor profile while altering the texture of foods like kimchi and yogurt.
Moreover, the balance of pH can affect how ingredients interact. A high pH can lead to softer, mushy textures in canned vegetables, while a lower pH helps maintain a firmer bite. This is particularly important in preserving fruits, where you want to retain their crispness and vibrant flavors.
Testing pH is Key to Success
Regularly measuring pH levels during food preservation ensures methods are effective and results are safe and delicious.
In conclusion, understanding the relationship between pH, flavor, and texture helps us create preserved foods that are not only safe but also delicious.
Final Thoughts on pH in Food Preservation
Understanding pH levels is crucial for anyone interested in food preservation. It informs us about the safety of our methods and the quality of the preserved foods we produce. By recognizing how pH affects various preservation techniques, we can choose the right approach for each food item, ensuring both safety and flavor.
As we've explored, whether you're canning tomatoes or fermenting cabbage, monitoring the pH level is key to achieving successful results. This knowledge empowers you to preserve your favorite foods while minimizing the risk of spoilage.

So, as you dive into the world of food preservation, remember that pH is your ally. Embrace it, test it, and let it guide you to delicious and safe homemade preserves.